NIST
The National Institute of Standards and Technology (NIST) hosts a data base of spectroscopic transitions for a variety of elements and their ions. This data base is an excellent tool for identifying spectral lines in stellar spectra. This article is a short introduction on how to use this web page.
Most important for our purpose is the upper part of this NIST web page, where one has to:
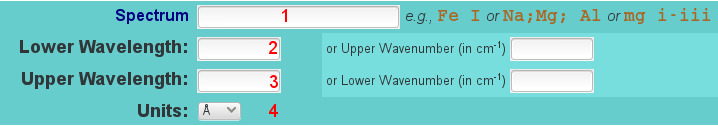
- enter the element symbol and optional the degree of ionization, otherwise the transitions from all ions will be displayed, keep it empty to search for all elements
- enter the lower value of the wavelength range of interest
- enter the upper value of the wavelength range of interest
- choose a unit (usually angstrom makes the most sense)
By clicking on the Retrieve Data button, a list with all spectral lines that fit the above criteria will be displayed.
Special remarks
Important note on the line identification: One has to roughly account for the abundances of the elements in the stellar atmospheres! For example rare earth elements have that name for a reason!
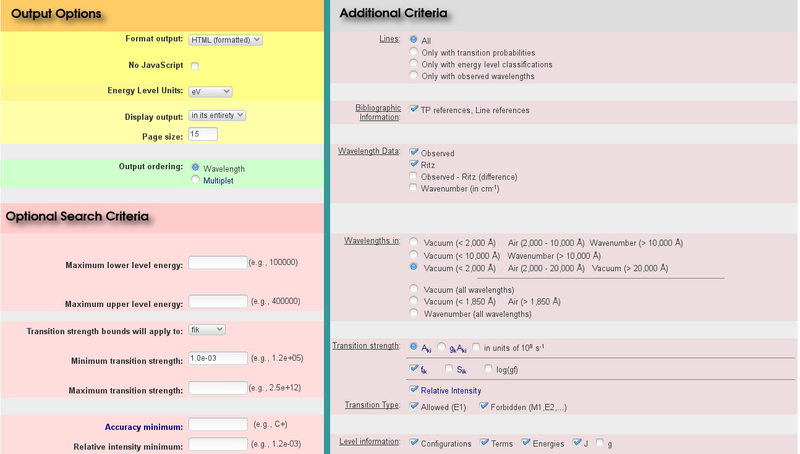
Furthermore, it is important to identify which of the presented transitions might be the one that is actually visible in the spectrum. For this purpose, it is helpful to also print the oscillator strength of all listed transitions. This can be achieved by activating the $f_{ik}$ option, which can be found via Additional Criteria → Transition Strength on the input page.
Moreover, one can and should restrict the oscillator strength to a reasonable range. With the exception of hydrogen and maybe helium, a line transition with an oscillator strength of less than $10^{-3}$ is unlikely to be visible in our spectra. A corresponding value needs to be entered in the Minimum transition strength field in the Optional Search Criteria box. Note that one also has to choose $f_{ik}$ in the above drop-down menu (““Transition strength bounds will apply to””).
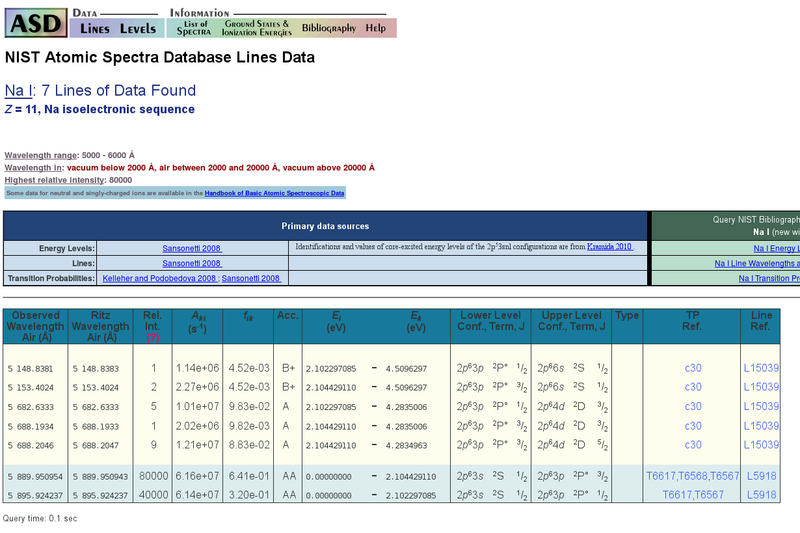
Example
In the following an example of neutral sodium is shown. In the wavelength range between $5000\,\unicode{x212B}$ and $6000\,\unicode{x212B}$ the well known sodium D-line doublet as well as further transitions with significantly lower oscillator strengths can be found. In the column Rel. Int., the relative intensities of the transitions are specified. Whether a line can be observed for a specific value of the relative intensity or not strongly depends on the investigated ion. Nevertheless, by comparing the intensities of different lines of the same ion the observability of a line can be evaluate.